Young DMD patients getting early access to life-changing drug at RJAH
Posted: 25 Sep 2025
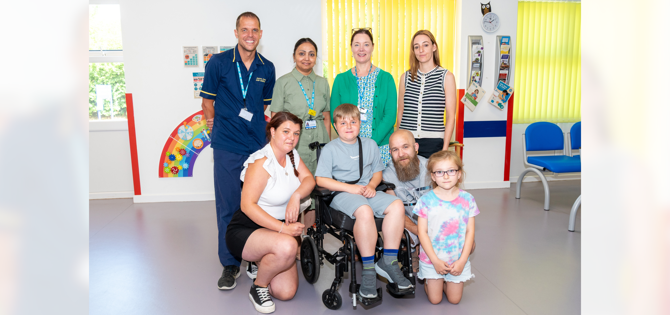
Young Duchenne Muscular Dystrophy (DMD) patients at The Robert Jones and Agnes Hunt Orthopaedic Hospital (RJAH) are among some of the first in the country to be getting access to a new drug called givinostat, which could slow the progression of their disease.
Givinostat, also known as Duvyzat, was conditionally approved by the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK in December 2024. However, it’s not yet available for routine use on the NHS. For this to happen, it must be recommended by the National Institute for Health and Care Excellence (NICE). NICE review how effective the treatment is and weigh it against the cost of the treatment. The process has started for givinostat, but a decision won’t be made until later in 2025.
However, the company which manufactures the drug is making it available free of charge to children and young people in the UK right now via an Early Access Programme (EAP) until the regulators make a decision about approval. RJAH is one of the first hospitals to have accessed this programme.
Professor Tracey Willis, Consultant Paediatric Neurologist with a Specialty Interest in Neuromuscular Disorders, at RJAH, said: "We now have eight patients being treated with givinostat through the EAP and we are continuing to roll it out to eligible patients.
"Following the successful clinical trial of givinostat, which we are proud to be a part of, we were aware of the EAP before it opened, so as soon as it became operational in the UK, we were ready to get going.
"Givinostat was quickly approved by our Drugs and Therapeutics Committee with no issues and having been one of the clinical trial sites, we were familiar with the medicine, so we were confident in making it available to our patients and understood what was required in terms of monitoring.
"Within just a few weeks, we were able to dose seven patients, whilst most tertiary hospitals were still in the process of organising a service delivery plan for patients," added Prof Willis. "The ‘can do’ attitude of the team, supported by management and an excellent pharmacy team has been key to enabling us to deliver this."
Ruben, aged 11, is one of the first DMD patients at RJAH to be receiving givnostat His mum Poppy said: "As a parent I feel extremely grateful that Ruben has been given the opportunity to start givinostat. It has filled me with hope for the future knowing that it will slow the progression of DMD down.
"Ruben will be able to continue doing the things he loves for longer such as playing football with his friends and being creative.
"It also gives more time for research, which fills me with hope that the future for our DMD community will be bright."